Electrochemical Sensors
In the Chair of Analytical Chemistry II, active research is carried out to develop chemical sensors that are 1) relevant to industrial/environmental applications and 2) economic for wide spread installation into various processes.
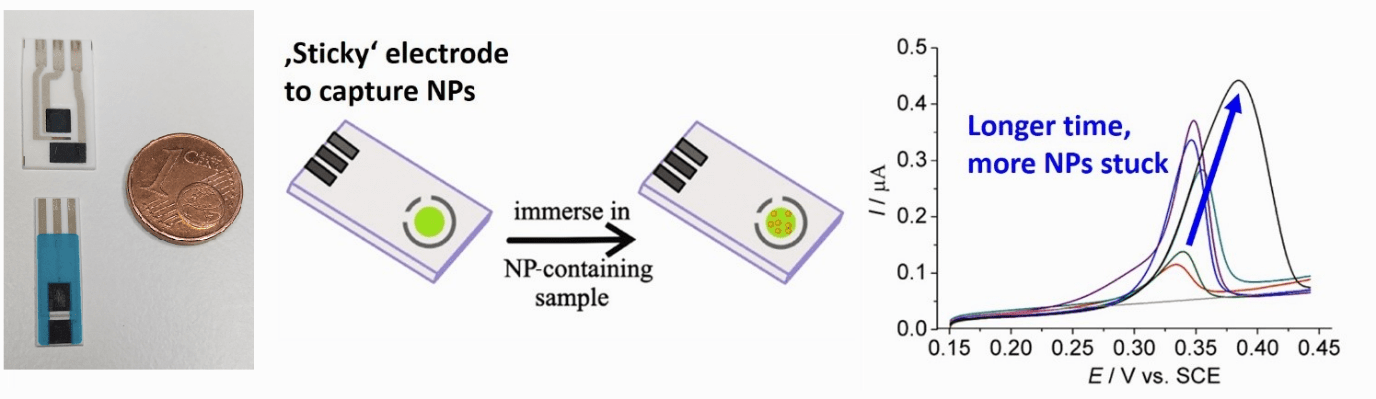
Detection of nanoparticles (NP) in sea water
Silver NPs are widely used as anti-bacterial disinfectant in many commercial products. However, upon release into the environment, silver NPs may potentially be hazardous to organisms due to the antimicrobial effect of Ag+. A major challenge of detecting NP in environmental sample is the low concentration of NP in such samples. Here the ‘sticky’ electrode approach we have developed to selectively pre-concentrate the silver NPs onto the electrode prior to their electrochemical quantification by Anodic Stripping Voltammetry. This allows us to measure particle concentrations down to femtomolar concentrations.
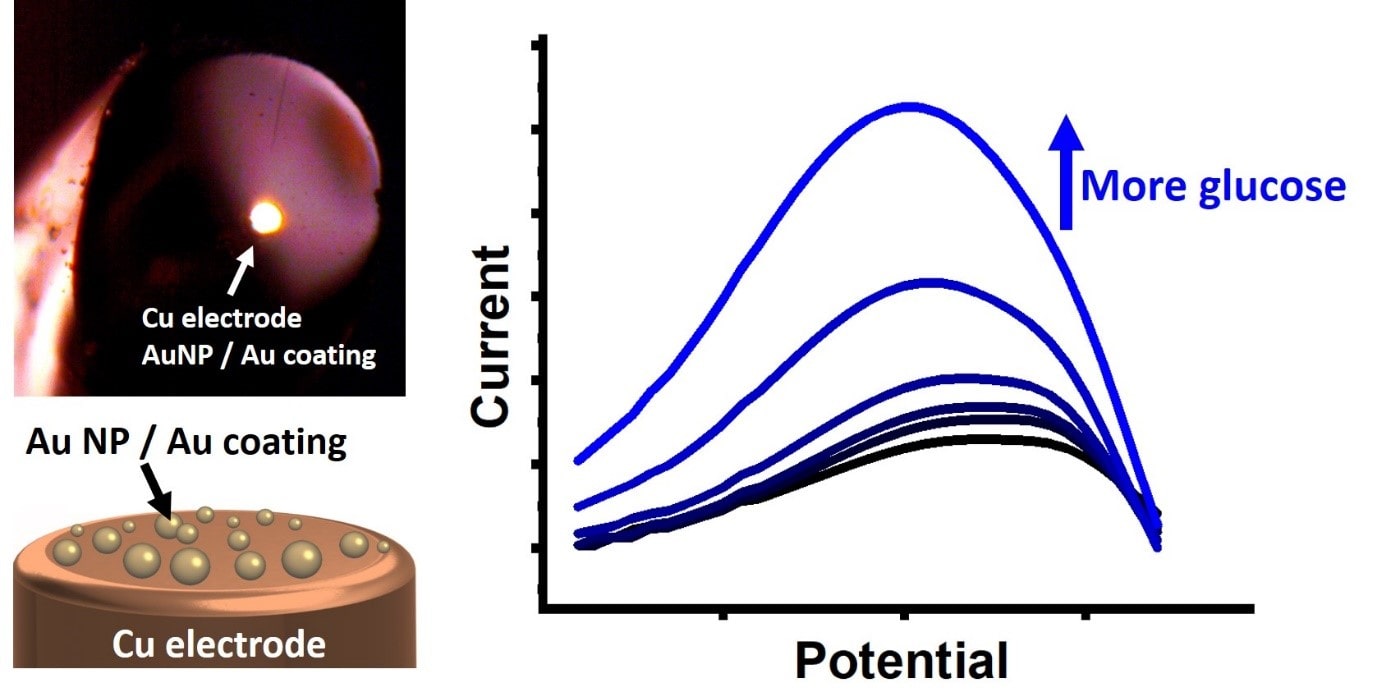
Glucose sensing and monitoring during industrial production processes
Being the molecule that powers most life forms on Earth, glucose is the subject for various sensors because of its widespread presence. We develop miniature and low-cost sensors for smart glucose sensing under industrial production conditions. These are based on copper wires as electrical contact and gold as a sensing material. This gold layer catalyses glucose oxidation, which results a quantitative signal that correlates to the glucose concentration. By using Au nanoparticles (AuNP) instead of fully coated Au layers, we significantly reduce sensor costs as a minimal amount of gold, and significantly decrease production.
Single nanoparticle electrochemistry for sensing
Our expertise in accurately measuring ultra-small electric currents, allows us to measure the current (and charge) resulting from the electrochemical transformation of individual nanoparticles. The associated currents and charge transferred between electrode and nanoparticle allows us to explore several properties of the nanoparticle, like their size, composition, concentration and agglomeration behaviour. By utilizing specific “indicator nanoparticles” and measuring the duration of the particle transformation, we have extended this approach to determine ion concentrations and ion mobility in different solvents. Alternatively, the composition of solvent mixtures can be assessed.
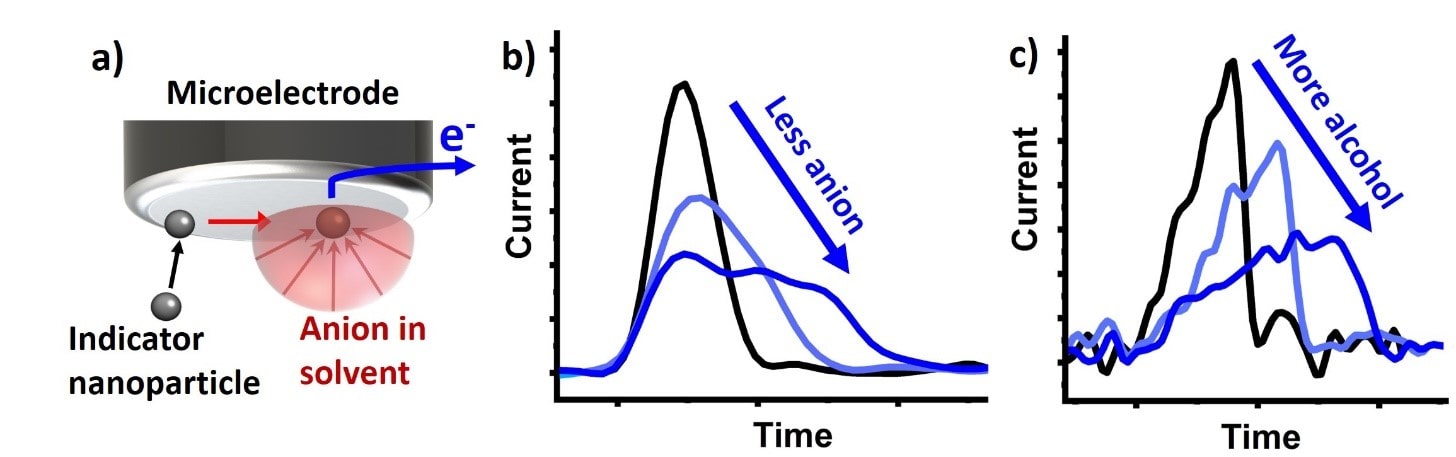
a) Indicator nanoparticle being electrified on a microelectrode, reacting with the surrounding surrounding anion, giving up electrons (e.g. AgNP + Cl– → AgCl + e–) which show up as a current peak in a current vs. time graph. b) If there is less anion in the solution, it takes more time for the indicator nanoparticle to fully react and resulting in a lengthened current peak. c) The lengthening of the current peak can also be seen, if the anion has reduced diffusion coefficient due to increased viscosity of the solvent by the addition of the alcohol.
Saber Print
Consortium leader: Kristina TschulikSABER PRINT is a cross-border collaboration project aimed at investigating the potential impact of smart (bio)-sensors on the process industry of the future. For more detailed information visit the SABER PRINT website.
A podcast interview from Quarks from 27.05.2021 (in german), in which the project and the background is explained, is available on the WDR website:
Partners:




